The development of sustainable mobility and new technologies opens up new scenarios for the future of humanity but also technical and logistical needs. From cars to drones and beyond: batteries are undoubtedly key players in this transition phase and the challenge for the future is to make them more durable and perform better.
A challenge that has been accepted and partly won by the Fluoritech Laboratory of the Department of Chemistry, Materials and Chemical Engineering “Giulio Natta” of the Politecnico di Milano, a laboratory led by Prof. Maurizio Sansotera who together with his team has patented LIFT Energy.
How the idea was born
LIFT Energy is a battery that takes advantage of fluorinated materials and their applications. When talking about batteries, one must always keep a clear distinction between primary batteries, which are the non-rechargeable ones, and secondary batteries, which are the rechargeable ones.
Lithium-ion batteries are among those that are rechargeable, while lithium metal batteries are non-rechargeable batteries. LIFT Energy’s goal is to make lithium metal compatible with “rechargeability.”
In so-called lithium-ion batteries, lithium is “diluted inside” an inert matrix in the form of lithium ion: since it is dispersed inside, it is less concentrated, and therefore with less charge for the same volume. Lithium metal, on the other hand, is energetically charged, without any diluent and with the charge concentrated in the so-called lithium metal lattice.
Until now, rechargeable lithium metal batteries could not be created because it during discharging and charging moves from anode to cathode and vice versa and follows current lines, preferential channels as if it were water.
The problem emerges during assembly as a laminette: the lithium after these charges tends to stack along these channels, creating pinnacles that as the battery goes on, grow to the point that they puncture the separator and bring the anode into contact with the cathode, causing a short circuit, with all its consequences: immediate discharge of all the energy and problems of overheating, fire or explosion, as the news reports on some lithium battery accidents confirm.
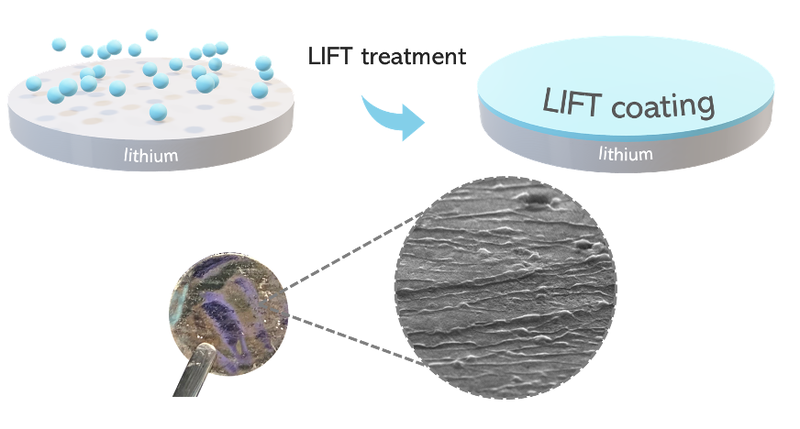
How it works
Researchers have reimagined the solid electrolyte interface (SEI), a solid interface between the electrode and the electrolyte already found in nature and already known to scientists. Artificial SEI is achieved by exposure of the Lithium metal electrode to a gaseous fluorinating agent.
By applying a fluorinated layer to the lithium path, the latter behaves like the ball in pachinko, a Japanese game resembling a vertical pinball machine, in which a series of pegs deflect it, preventing its descent from always occurring in the same place. Similarly, lithium is deflected and never returns to the same spot, inhibiting the formation of pincushions.
By engineering the formation of the SEI, researchers have given the lithium “levees,” as if it were a river whose course is diverted from its natural bank to prevent dangerous “flooding.” In the case of the battery, the danger is the accumulation of lithium leading to overheating and explosion.

From idea to patent
Maurizio Sansotera and his staff at first participated in Polihub’s Switch 2 Product business acceleration program, which helps to enhance laboratory ideas to see if they can be of industrial interest. They won the ENI Joule award, which allowed them to conduct the first small investments for electrochemical characterizations of their system and then assembled batteries with this type of technology inside.
The process of enhancing the technology continued with the patent, which also allowed them to present the technology to potential investors.
Through scientific collaborations, the team found an interested investor and signed an investment contract that led to the development of a prototype battery for use in a demonstrator device.
Because of the characteristics of the technology, the researchers envisioned the drone as a tool to test the effects of their batteries because the higher charge density allows them to shrink by 50 percent for the same charge and dedicate the saved weight to increase transportable mass. Alternatively for the same volume the batteries can have twice the charge and the drone gains in flight range.
The challenge now is to get those investments that will be needed to move from prototype to first production scale of a series of battery batches, to be tested then by industrial entities that do the assembly of the actual batteries instead.
The applications and benefits
“Surely electric mobility is an important field of application,” comments Prof. Maurizio Sansotera- There even more investments are needed because while at present the drone market is a bit of a production niche let’s say, the electric car market is a very large market and consequently requires a structure side by side with production that is large enough to guarantee the production capacity that the car market needs”.
The idea is to be able to create mini-cars that can incorporate the technology. Looking also at other case studies around the world in Europe but also in America and Asia, LIFT Energy can make a difference because it gives a real competitive advantage to those who would like to invest in a new technology that, among other things, is compatible with the other major technology trends that are in the battery market today. Three examples out of all: it is compatible with lithium-sulfur, high-energy cathodes that are being developed at the moment; it is compatible with all solid electrolyte technologies, lithium metal being precisely a solid component; and it is also compatible treatment with sodium batteries, which is another important issue, because while lithium has geopolitical constraints due to its uneven distribution around the world, sodium, being present in the sea is much more easily available to most states in the world.

