Femur fracture is a familiar experience in our daily perception, within a society whose average age is rising more and more. Its surgical treatment is one of the most widespread medical practices in Italy, and as such raises some questions in terms of sustainability.
Have we ever reflected on what the environmental and social impact of a surgical operation is? This was done by the research group of PRESTO, a Polisocial project resulting from the joint multidisciplinary work of three departments of the Politecnico di Milano: Management, Economics and Industrial Engineering; Mechanics; Electronics, Information and Bioengineering.
The project first measured the extent of the environmental and social impact of these fractures, subsequently developing innovative technological solutions to prevent and improve the treatment of femur fractures in people with bone fragility, such as those suffering from osteoporosis.
I met researchers from the various working groups that took part in the project, who told me how they achieved their excellent results and the concrete benefits that this research will bring, both for the health of patients and for the sustainability of health systems in the long term.
The Management, Economics and Industrial Engineering team
My journey begins with the researchers of the management engineering group PLA.NET, focused on designing more sustainable supply chains, with whom we find ourselves online.
The team measured the extent of the environmental and social impact associated with the bone fractures under study.
I usually speak to a single representative of the research team, but this time all four answered the call. And the impression is precisely that of a cohesive and close-knit group.
Professor Margherita Pero coordinates the team, which includes Professor Federica Ciccullo and PhD students Francesca Bianchi and Eleonora Catellani.

Professor Pero, how did the idea from which your research project was born come about?
Our research group deals with supply chain studies, i.e. the design, planning and management of supply chains. We have expertise in terms of impact measurement, sustainability of supply chains.
It was the first time we dealt with the medical context, because in fact we work in sectors that are also very different, but always with a measure-oriented approach.
In this case, we asked ourselves the question: in a country like ours, what is the environmental and social impact that the prevention and treatment of bone fractures currently has? And what can we do, both on the prevention and treatment side, to reduce this double impact?
Where did you start from in this analysis?
We started from the literature, where quite predictably we had confirmation that the healthcare sector has a negative impact on the environment.
It is a highly polluting sector because it produces a lot of waste that clearly needs to be treated in a specific way, typically burned. It is also a sector that consumes a lot of energy to run all the machinery and systems we use. And often this energy is produced with fossil fuels.
Also in the literature, we have verified that the treatment of femur fracture is, in the orthopedic sector, one of the most impactful, especially in the operating room.
What about the social impact?
We asked ourselves what the cost paid by patients and the community was, both at the level of intervention, which is the most visible side, and that of post-surgery, which is the least studied aspect.
What figures are we talking about, Dr. Bianchi?
In 2017, there were 560,000 fragility fractures in Italy, and the annual incidence is expected to increase to 690,000 by 2030.
Although femur fractures account for 1/5 of total fractures, they are estimated to account for 59% of the total costs associated with fractures.
In 2017, the economic burden associated with fractures was around €9.4 billion, set to grow to €11.9 billion in 2030.
These are impressive numbers, and your project wants to affect these numbers by following various lines of action. Let’s start with the environmental impact, Dr. Catellani?
As we said, while studies on the economic impact had already been done, the environmental and social impact remained a little less investigated and so we focused on this part.
The literature already photographed how the health sector is very impactful, both in terms of waste, where the use of single-use is widespread, and energy consumption, due to the energy-intensive machinery in operating rooms.
In addition, it is an area where it is difficult to recycle, because the disposal of medical waste is strictly regulated.
Starting from this state of affairs, how did you move?
What we did was to start with interviews, both with the operators, then with the surgeons, and with the scientific director of the Galeazzi Hospital.
We have carried out a mapping of the process in which we have distinguished various phases in the entire patient journey, from emergency room to rehabilitation. All this to identify the sources of environmental impact, including energy, materials, water consumption, etc.
From the interviews, which were later validated, we understood that it made sense to focus on surgery, because it was the one with the most impact in percentage terms.

Your work was not limited to interviews, but also took place in the field, right?
Yes, we went to see some interventions, to understand mainly where the waste was generated from. And we understood that they are divided into three categories: sharps, paper and plastics, medical waste.
We then tried to quantify this waste: on the one hand we have data on water and energy consumption, which we took by validating the literature, while in the hospital we focused on materials, weighing the waste that came out of the operating room when a femur was operated.
What were the results of these measures?
It emerged that for a one-hour operation, about 40 kW/h is needed to power the operating room and an average of 6 kg of medical waste is produced with forced disposal in the incinerator.
Dr. Bianchi, shall we move on to the phase of social impact detection?
The social impact is the one that can be detected mainly in the post-operative phase, from the moment there is hospital discharge, in which the patient finds himself living a new everyday life. This aspect is not particularly covered in the literature, especially in relation to femoral fractures.
The impact actually exists, and concerns social relationships, the patient’s autonomy in daily life: shopping, cooking, cleaning or even trivially getting dressed. And then the possibility of going out independently, meeting friends, family, taking a simple walk.
How did you manage to “quantify” it?
We first identified which stakeholders were impacted: not only the patient himself, but also the caregivers around him, who may be professional or already part of the patient’s family or community.
We then identified the indicators and tried to quantify them both in quantitative and qualitative terms through a method that included both interviews and questionnaires, aimed at patients and family members.
The questionnaire asked precisely how many hours were dedicated to the patient weekly, and to evaluate on a qualitative scale what was the decrease in his autonomy.
Further interviews were then carried out with the health staff of the RSAs and physiotherapists at home, who live their daily lives together with the patients. For this activity we had the precious involvement of Cittandinanzattiva, a patient association.
What data emerged?
We found that the fracture significantly limited the daily activities of the vast majority of patients, and to a large extent also social ones. The hours dedicated to the care of the majority of patients by caregivers reaches or exceeds the equivalent of three days a week. The total expense incurred for the assistance of femur surgery exceeds 1,000 euros in most cases.
From the point of view of autonomy, patients tell of the difficulties in having to depend entirely on their loved ones following hospital discharge and the limitations in daily activities. Home physiotherapists have brought out the psychological element of the signs of disability: the cane or wheelchair somehow inhibits the elderly person from going outside because he does not want to show weakness.
Professor Ciccullo, how did you develop impact mitigation strategies?
Starting from the information obtained from the interviews, we divided the possible solutions into three macro-phases of the process: pre-fracture, fracture and post-fracture. We focused on the pre and post phases.
For pre-fracture, we have been shown different strategies to recognize the risk factors for different patients. The main risk factor is osteoporosis. But there are also factors of a more behavioral nature, such as the fact that the patient is particularly sedentary, that he leads a lifestyle that in some way then favors exposure to a fracture.
We also identified strategies to reduce exposure to the risk of falling in the home environment.
There is also the issue related to diagnostics, to act on the timely identification of the patient’s particular exposure to certain risk factors.

For the post-operative phase, however?
Possible strategies have been reported to us to speed up the treatment and the post-operative course. One of these is precisely the technology based on micro-constructs that is developed within the project.
An important issue on which we were asked to investigate is that of the extra costs that families have to incur because the RSAs do not have dedicated equipment for diagnostic imaging and therefore have to transfer the patient to the hospital with costs borne by the patient himself.
Then there are the costs of prescribing aids necessary for walking, which are not always covered by the national health system.
What about waste management, which we were talking about earlier?
Talking again with healthcare personnel, we tried to identify some strategies that could help reduce waste production in the operating room.
We can work on reducing packaging, which is currently very abundant. In addition, sterilization and reuse of cutting edges can be re-evaluated compared to the current massive use of disposable instruments.
Another strategy is to introduce recycling, given that separate waste collection is not currently carried out in the operating room.
Have you received demonstrations of interest from healthcare facilities in your model?
At the moment, the Galeazzi Hospital, thanks to whose collaboration we carried out the study, has shown interest in continuing the work of analyzing the environmental impact of operating rooms.
So we are trying to go ahead with the activity, also proposing a degree thesis aimed at supporting us in data collection. A more detailed analysis of the waste generated and the practices that generate this waste is now needed, to understand how to deal with it.
The Mechanics team
A few days later I arrive at the Bovisa Campus, where the Department of Mechanics is located. Here I meet Federica Buccino, the researcher who, together with Professor Laura Vergani, delved into the part on patient-specific structures to deal with fragility fractures.
After a degree in biomedical engineering and a master’s degree in biomechanics, she entered the Alta Scuola Politecnica, where she became passionate about the topic of bones and met Laura Vergani.
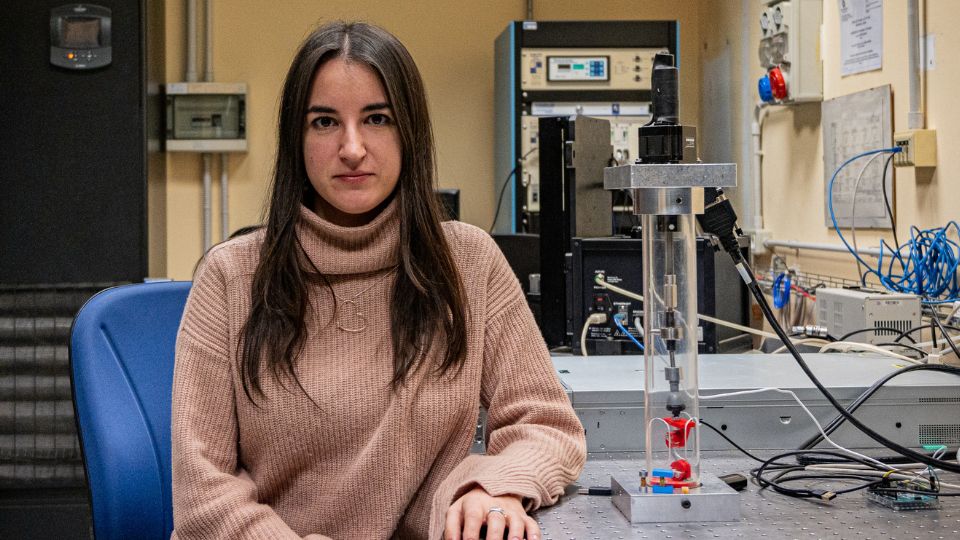
How did your collaboration come about?
Professor Vergani had worked on bovine samples in the past and collaborated with a research group at ETH Zurich.
Combining his knowledge of fracture mechanics with my interest in the biomechanical field, the idea of understanding the origin of bone fractures emerged. I did a research period at ETH, where I deepened my knowledge on damage phenomena at small scales.
This is how the idea of my PhD with the teacher began: we wanted to find a way to implement a predictive approach for fracture management.
The beginning was not easy, was it?
My PhD began right at the same time as Covid-19. And so I found myself staying in Milan. The ethics committee was still in the drafting phase, so, with a number of enthusiastic thesis students, we began to build a test setup compatible with ultra-high-resolution imaging techniques, such as synchrotron. While waiting for the bone samples, we started testing the setup on the nutshells.
Covid, however, has also allowed our research team to make some interesting discoveries. We verified that bone samples considered as healthy that had contracted covid within a year of the fracture had micro-morphological characteristics similar to osteoporotic patients.
The path then continued successfully, and today it has led you to the position of researcher at the Department of Mechanics.
My main research activity focuses on the mechanisms of damage in biological materials and the design of new bioinspired materials thanks to artificial intelligence and other innovative techniques.
In practice, I study nature as a source of design for new materials: composites, metamaterials, with improved characteristics thanks to the in-depth study of the toughening mechanisms present in natural materials such as bone.
As you can see, Polisocial was not a call in which we participated by chance, but it is a reflection of my background and well-aligned projects in this regard.
As for PRESTO, what is the role of mechanics in reducing the impact of surgeries?
We have worked following two main guidelines, that of prevention and that of treatment.
To reduce the eco-clinical-social impact, we wanted to adopt a strategy that we consider innovative: while the clinic currently focuses on macro and mesoscale, we wanted to look at what happens at small scales.
Can you explain a little better what this means?
Sure. If the large scale is that of the bone as a whole, the mesoscale is the one that focuses on the trabecular (porous) and cortical (denser) bone. At the microscale the bone is composed of “voids” called gaps. In reality, they are not really empty, because inside them there are osteocytes, the cells responsible for bone remodelling.
What was your intuition?
The clinical observation currently in use focuses on macro and mesoscale. Techniques such as X-rays and DEXA bone densitometry make it possible to investigate what happens to the bone up to its mesoscale. It is observed that, in the presence of bone pathologies or due to aging, the bone becomes more porotic and becomes brittle. But in this way, only 70% of fractures can be identified. In 30% of cases, the fracture is not identified and after some time the patient will find himself in the hospital with a much more critical fracture. This is especially problematic in elderly patients, where a fracture can lead to bed rest and loss of mobility and autonomy.
The idea behind our contribution to PRESTO was born to solve this critical issue. We decided to look at the small scale and make clinical deductions on it, to try to understand the presence of fractures already at the microscale and cover that 30% of clinical “unknown”.

What did you understand by examining the bones at the very small scale?
We realized that these very small ellipsoidal porosities, the gaps, connected to each other by canaliculi, change shape and density in the presence of bone pathologies, reflecting different ways of fracture propagation.
In older people, the gaps become more spherical. In this way, the bone fracture is no longer deflected by these gaps, but speeds up its propagation process.
This is a very interesting mechanism, which we asked ourselves to understand if useful information could be obtained from an observation of this type for clinical practice.
And what imaging technique did you focus on?
Among those currently in use, the only clinical technique capable of observing the gaps today is histology. And we said to ourselves: why not increase the histology that works on two dimensions with the 3D resolution of the synchrotron?
This is where the bioengineering team entered the process to process the enormous amount of data, one terabyte, which constitutes the image provided by the synchrotron for a single bone sample.
What is synchrotron, for the uninitiated?
A synchrotron is a type of particle accelerator in which electrons are accelerated to speeds close to the speed of light along a closed loop, called a synchrotron ring. During their path, magnetic fields curve the trajectory of electrons, which, due to this deviation, emit high-intensity electromagnetic radiation, known as synchrotron radiation.
This radiation covers a wide spectrum of wavelengths (from X-rays to infrared) and is extremely collimated and bright, making it a very powerful tool for imaging and analyzing molecular materials and structures with very high spatial resolution and sensitivity.
Let’s get to the second strand of your approach…
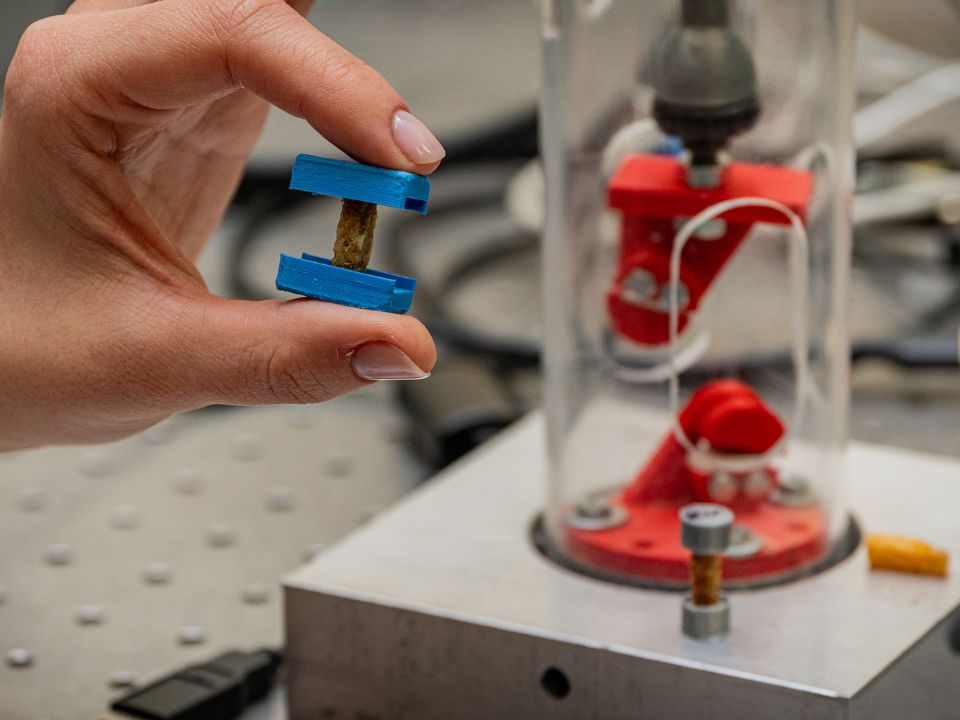
After addressing the role of prevention, we turned to treatment. And when there is nothing to do but treat, i.e. in the presence of extensive bone defects, our attention has gone to the design of new supports, called scaffolds, to regenerate already critical fractures.
A scaffold is a three-dimensional structure, “a biological scaffold” used to support the growth and regeneration of tissues or organs.
If healthy bone functions as a system of interconnected pores, why not use the same information about adequate porosity that we derived from the microscale study to make scaffolds with optimal porosity?
Thanks to the 3D images of Elettra-Sincrotone in Trieste, we were able to print scaffolds in biocompatible material to be inserted into the human body, with the same porosity as the mesoscale, but also with micro porosity, including gaps and canaliculi. An idea thanks to which we have created these microconstructs that we are cellularizing in vitro.

How is the development going?
We have seen from some preliminary evidence that bone cells are particularly tempted to penetrate this architecture, because it is very similar to the natural one.
By extrapolating the fundamental data, we were able to design a multiscale construct, whose design works much better than the usually used constructs, often characterized by limited cell penetration and differentiation.
What are the consequences of the two approaches on impact?
Our goal is, through early diagnosis and personalized treatment, to minimize the extensive surgical treatment, i.e. hip prosthesis, avoiding the great criticality of the intervention and its ecological impact.
In terms of environmental impact too, I want to emphasize that we use totally biodegradable materials. For example, we have also worked with silk fibroin, which is part of a circular economy.
It is precisely digital fabrication that allows us to minimize waste: we are able to place sustainable material in three-dimensional space exactly where the patient-specific design envisages.
Summarizing our perspective of minimizing waste in three points: digital design and AI processing; digital fabrication with 3D printing and control in the use of material and shape; biodegradable materials from biological waste.
Was it an advantage to bring together such a multidisciplinary group?
The proposal of the PRESTO group was born in response to the Polisocial 2022 call, which had as its theme “Local Development and Ecological Transition”. The spirit of the Polisocial calls is precisely to bring together skills from several departments of the Politecnico to do research of social utility.
Polisocial was the seed fund that kicked off joint activities, both from the point of view of calls and from the point of view of doctoral scholarships.
I would like to say that the collaboration was a success, an interpenetration of knowledge that was fundamental to give life to the project. We have combined the experience and expertise of three different research groups that have been working for years on compatible topics, but without ever having worked together on a project of this caliber.
Very interesting are also the participations in conferences involving the various research groups.
Once the project is finished, how is your business continuing?
The project has officially ended, but the closing event of PRESTO was a new beginning. The activity of the whole team is continuing in terms of research and planning to participate in new calls.
Our work of measuring biological waste with management engineers is continuing, and then we are thinking about a strategy to plan the minimization of waste. We are still working on the guidelines with the Galeazzi Hospital.
Continuing to work with partners is important to you…
In addition to the scientific interest of clinicians, we want to expand social interest with the participation of RSAs. This is why we are opening new dialogues for the participation of these figures.
We were particularly surprised by the great participation of the actors involved, departments and hospital. Among other things, the partners collaborate without funding, and despite this, participation is particularly relevant, because it guarantees the growth of the project in the immediate future.
Paraphrasing the name of the project, we need to act QUICKLY: act immediately in dealing with this type of fracture.
The Computer Sciences and Bioengineering team
Eleonora D’Arnese is a biomedical engineer. After a successful career at the Polytechnic, he is now a lecturer at the University of Edinburgh. It is from there that he connects for our online chat, the last piece of my meetings with the various souls of the PRESTO project.

Today you are in Edinburgh, but your path at the Polytechnic was fundamental. How did it unfold?
It was in the second year of my bachelor’s degree that I met Marco Santambrogio and the NECST Lab, a laboratory of the Department of Electronics, Computer Science and Bioengineering where many different lines of research coexist.
In those years, many biomedical enthusiasts had found themselves there. We brought our knowledge to a laboratory with a quite different background, and I must say that this hybridization has been positive: over the years many new projects and professional figures have emerged.
After my double master’s degree in Chicago at UIC, I took up the challenge of a doctorate in computer engineering at the Politecnico di Milano, but always with the strong desire to devote myself to the analysis of biomedical images, precisely because of their impact.
The PRESTO project represented my postdoctoral research period at the Polytechnic.
How was your team formed?
In addition to Marco Santambrogio and me, there is Isabella Poles in the team, with whom we have built a nice group focused on imaging, open to different types of collaborations.
How did you get into the PRESTO project?
Starting from the great work that the researchers of the Department of Mechanics were doing.
They wanted to develop injectable scaffolds that would facilitate fracture regeneration. Because then when the bone breaks it is difficult for it to weld well afterwards, and it is more likely that there will be other fractures. Injecting these active scaffolds into the fracture area would allow it to be treated better, reducing the likelihood of re-fracture. A problem had arisen at the level of the calculations to be carried out, which were very heavy and laborious. This limitation allowed them to analyze subsamples of limited size. This is where the idea was born, in 2022, to collaborate with our team, to try to see if it was actually possible to automate the analysis with artificial intelligence more effectively, and also to verify whether artificial intelligence could reach the same conclusion as biomechanical simulations.
What was your approach?
What we actually did was to approach the problem from two perspectives: on the one hand, to facilitate the work of mechanics in support of the current flow; on the other hand, try a parallel flow to see if the results could match.
How did you put it into practice?
We analyzed the mechanisms of bone damage at the microscale level, using very high-resolution images obtained by subjecting bone samples to controlled efforts at the Elettra Sincrotrone Trieste.
They are gigantic images of very small samples of femoral heads, with a resolution of the order of microns. We processed them through advanced artificial intelligence techniques to classify and segment the regions of interest, extract morphological parameters indicative of the state of bone health, allowing the mechanical department to carry out biomechanical simulations on a larger scale capable of predicting the behavior of tissues under mechanical loads.
What parameters did you evaluate in these samples?
Mechanical engineers told us that stress adaptation is one of the most interesting parameters for simulations.
This parameter is used to try to understand how the bone would actually respond under stress.

How did you analyze these samples?
What we tried to do in the first instance was to try to segment the gaps, the small holes in the bone where the cells that allow the bone to regenerate live.
It was extremely tiring for them to do this manually, because in a single image there are thousands of these holes, and each sample is represented by more than 2,000 very large images: it was therefore not feasible.
What we did was create an artificial intelligence model that would segment all these gaps on its own. Initially we did it collaboratively, because the manual annotations that the mechanics gave us for some images helped.
We then built a model that was accurate enough for them, which we gradually refined more and more.
What was your goal in the second phase?
We wanted to create a model that could tell us whether a piece of bone was osteoporotic or healthy, without having to go through mechanical simulation, i.e. directly, fully automatically.
It was a challenge, actually, because at the beginning it seemed like there was nothing in these images that allowed us to understand it.
What was the turning point?
We realized that we had posed ourselves badly with respect to the problem to be solved.
A doctor from these images is not able to tell whether the sample is healthy or sick, and so we naively, believing the opposite, were convinced that the model should have identified the differences between the two cases.
We later discovered that this was not actually the case, and that therefore we needed to deepen our knowledge of the characteristics of these images. Since these gaps are very important from a mechanical point of view, we have also tried to make them relevant for our artificial intelligence model.
Were the results encouraging?
Yes, because we realized that by “stressing” the model, it began to learn to distinguish healthy patients from osteoporotic patients from gaps.
It was a very positive result both from a bioengineering and a mechanical point of view, because it substantially supported the mechanical simulations, in corroborating the fact that they were the gaps that brought information on the presence of osteoporosis.
At that point the question we asked ourselves was whether it was possible for an entire piece of bone to be completely osteoporotic. Ideally not, because otherwise a person would not even be standing. But we had a single piece of information for the whole piece of bone.
At that point, how did you move?
We went to make our model a little more complex, trying to understand which areas of the bone were actually osteoporotic and those actually healthy, and trying to use only those as indicators.
We assumed that in the sample there was a co-presence with healthy births even in the case of osteoporosis. And so with the second model, where we extracted only the areas where we were sure there was osteoporosis, performance increased.
In a sense, could we say that artificial intelligence, in this case, can understand something that the doctor, the human person, cannot understand?
Let’s say that the doctor or expert works on another scale of images. To diagnose osteoporosis, they use X-rays or DEXA bone densitometry, then full-scale images of the person.
The problem is that when osteoporosis reaches the state in which it is visible with DEXA or X-rays, it means that it is already present, while we really wanted to find methods for an earlier diagnosis.
When we begin to understand what are the actual parameters that have a correlation with osteoporosis, we would then be able to diagnose it earlier in the future.
What is the challenge?
The open questions are perhaps more challenging: finding a way to understand how to move from our microscale analysis to macro-scale images.
We cannot afford to take a bone sample from every person to understand if osteoporosis is about to develop. The vision I like to adopt is to try to understand how to combine what we have learned from this project with today’s clinical practice.
We were able to find meaning in our model, thanks to reasonable results. The main result is precisely that we found a concordance with the mechanical simulations.
Another big question is: how do we do with the imaging present in the clinic today to prevent what we have learned from this project?
What other baggage do you take away from this project?
There was great teamwork in our team, with Isabella Poles and Marco Santambrogio. Isabella’s work was truly remarkable and fundamental in order to achieve these results in a year and a half, also recognized at the level of publications.
In addition, our model proved to be not only functional to the work that mechanics are doing in this specific case, but autonomous and applicable in other cases.
I also really appreciated the collaboration that has been established between departments that may seem a bit distant, but which have worked very well together, creating a positive impact.
Are you going on working on these issues?
As SOON the project is officially over, but there is certainly the will to try to continue this fruitful collaboration, to see where it takes us.
