In April this year, two researchers from the Politecnico di Milano have received the ERC Advanced Grant, funding assigned by the European Research Council (ERC) to academics who are accomplished in their field, to conduct innovative and high-risk projects.
This type of European funding is highly coveted: in order to receive it you must outperform the competition in a very competitive selection process. This year, for example, two of our professors distinguished themselves amongst the 1,735 projects submitted, of which only 14.6% were successful.
We have met with the first of these two researchers, professor Manuela Raimondi from the Department of Chemistry, Materials and Chemical Engineering “Giulio Natta”. She will work on the BEACONSANDEGG project which combines bioengineering, oncology, genetics, microtechnology, biophysics and pharmacology. The aim is to develop an innovative platform capable of replicating tumour progression in the laboratory by exploiting the vascularisation of a living organism.

Good afternoon professor Raimondi. Let’s start with a presentation of your research group.
We are the Mechanobiology group from Politecnico di Milano, and we are, first and foremost, bioengineers. Research is our passion. Researchers of all levels work together with PhD students and graduate candidates in the labs.
Here at the Mechanobiology lab we work on both experimental and computational models which reproduce biological functions. We have been doing this for 20 years, studying various aspects of body’s functions.
In a sentence, what is Mechanobiology?
Mechanobiology is the field of science which studies the influence of physical forces on biological response. For example, the influence of physical forces on the formation and healing of a tissue, or the influence of forces on the adhesion between cells in a living tissue. It is traditional biomechanics on a microscopic scale.
Tell us about the BEACONSANDEGG project
We propose to build a model of how a phenomenon linked to the progression of breast cancer, that is tumour fibrosis, occurs. We also refer to this phenomenon as fibrotic induration because it is characterised by the formation of a stiff tissue such as that formed in lumps in the breast.
Why is it so important to study fibrotic induration?
The occurrence of fibrotic induration, a complex phenomenon which involves an immune reaction, can indicate tumour progression. In breast cancer, aggressiveness is related to fibrotic stiffening of the tumour tissue.
Due to fibrotic induration, it is progressively harder for chemotherapy drugs to target cancer cells: the drugs reach the tumour through the bloodstream but cannot reach the cells because the fibrotic tissue increasingly acts as a physical barrier to their penetrating the tissue. It creates a sort of pharmaceutical resistance, which in the last instance is what causes tumour progression.
What are the difficulties of studying this phenomenon?
You cannot simply put the cells in a culture dish in order to reproduce fibrosis. We’re talking about a complex phenomenon in which the patient’s blood vessels and immune system are taking part.
The main difficulty in creating a cellular model of fibrosis is that the hierarchy and the permeability of the tumour’s vascular network cannot be replicated in a culture. Moreover, fibrosis is very difficult to reproduce because it’s a slow process which can take months, and it’s also impossible to study an animal model for such a long time. In the same patients, it can take years to be able to observe it.

This is where the idea for your model comes from. What are the characteristics which make it innovative?
The idea was to build a model of fibrotic induration which was halfway between a cellular model in a culture and an animal model.
In practice, we model micro-tumours at varying stages of fibrosis. We will use human breast cancer cells adhered to 3D polymeric micro-plates. The micro-tumours will be implanted in the respiratory membrane of embryonated eggs in order to elicit a fibrotic foreign body reaction in the micro-tumours. This is a fibrotic induration which is very similar to that which occurs in the tumour, and can then be adapted to our aim to build a model of this phenomenon.
We deliberately generate these micro-tumour models in varying stages of fibrosis, then we inject an antitumour drug into the embryo’s circulatory system and examine the barrier effect on the drug, which causes different cell death in different micro-tumours.
With a microscope, we can see the blood vessels and the collagen inside the micro-tumours, which are the fundamental constituents of a fibrotic reaction, like in scars. The more collagen we find, the denser and more oriented it is, the more difficult it will be for the chemotherapy drugs to reach the cells through the bloodstream.
Furthermore, we will manipulate the geometry of the polymeric micro-supports to condition the infiltration of the micro-tumours by the vessels and cells of the embryo.
What will be the next phase in building the model?
The tumour fibrosis model will be validated by testing its druggability, that is, how the drugs affect the tumour cells within the fibrotic microenvironment. We will test several approved antitumour drugs on the model, the clinical outcome of which is known to depend on the level of tumour fibrosis.
It will also provide a standardisable and ethical platform to promote the clinical translation of new therapeutic products in oncology.
In fact, your model provides a solution to several criticalities of other methodologies in terms of ethics…
What we’re doing is opening a window through which we can see directly the tumour fibrosis progression in a living organism. However, the embryonic avian egg is not considered an animal from a regulatory standpoint. Our method is therefore much more ethically acceptable, in comparison to animals traditionally used in experiments.
The eggs that we use are fertilised by a rooster, with the embryo inside. It’s a research method used by many laboratories and universities, like at that Politecnico, worldwide, where there aren’t the facilities to handle test animals.

Where did the name BEACONSANDEGGS come from?
Eggs we’ve already discussed… ‘Beacon’ means a bright landmark or reference point, like a lighthouse.
Beacon is also a term commonly used in biophysics to refer to fluorescent tags which are used in microscopic observations, to orient oneself in time and space, in order to follow a process taking place in the organism.
That is what we do, implanting into the chicken embryo the structure with tumour cells and then putting the whole system under the microscope, to follow the evolution of fibrosis over time.
The beacons form a regular 3D structure that provides spatial reference points in the various stages. At each stage we know exactly the distance between the various beacons, also in height. We need precisely these spatial reference points to be able to, over time, reposition the microscope’s field of view, and to be able to correct any aberrations which might occur due to the fact that we’re studying in depth a living organism which evolves very quickly.
The structure that contains the tumour cells also includes these reference points, the beacons, which enable us to reconstruct a computational model outside of the experimental system thus allowing us to predict the concentration of chemotherapy drugs that will reach the cells as a function of the degree of fibrosis.
The current project has distant roots…
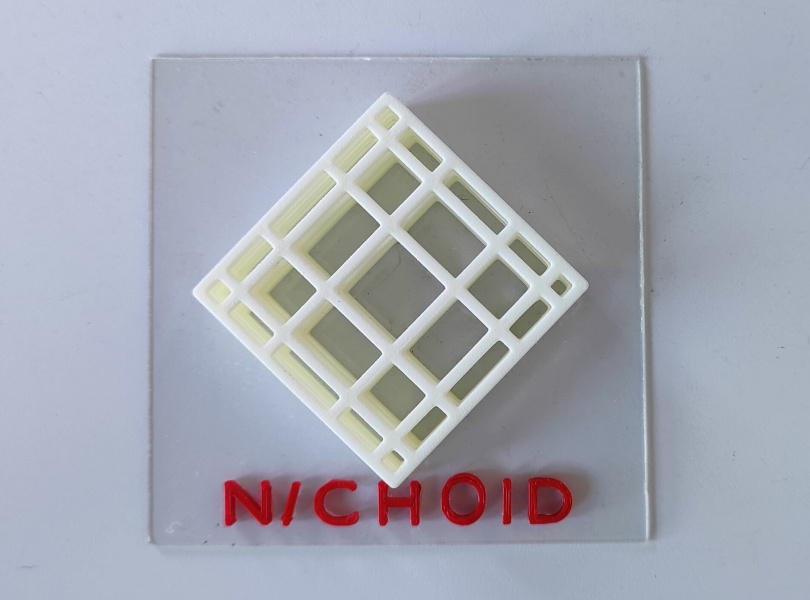
A previous ERC project which I coordinated, called NICHOID, was fundamental. It took place over the last few years and succeeded in getting the idea across that with artificial micro-structures you could simulate a complex biological phenomenon in a model, external to the human body. During that project, the complex biological phenomenon that I modelled was the microenvironment of mesenchymal stem cells, in other words, the cells which become our musculoskeletal connective tissue.
We started by making the hypothesis that, thanks to these microstructures, we could build a model of a natural stem cell niche outside of the human body, and we succeeded.
Many of our publications have demonstrated that the stem cells, living and dividing attached to the artificial microstructures, maintain a functionality very similar to that which they have in their natural niches in our body. And this paved the way for modelling of other complex niches, such as the fibrotic tumour niche.
Could you please explain the concept of the niche?
By niche we mean a microenvironment, we’re talking about a scale of only a few cells. Everything that we do in the laboratory can’t be seen by the naked eye. That’s why we work so much with colleagues in the Physics Department, and there is a structured biophysicist amongst us who handles the microscopy and fluorescence part.
Emboldened by this new niche expertise, we wanted to take it to the next level by dealing with another, increasingly complex niche, the fibrotic niche of solid tumours.
I wouldn’t be here if they hadn’t funded NICHOID, and another two related ERC projects on technology transfer. Five years ago we started from three microstructures and today we can make 8,000 of them within 24 hours thanks to the technological base we have developed.
The nichoid idea was also successful outside of the Politecnico…
The nichoid is already on the market thanks to a start-up which we founded in 2019. It took 12 years and three ERC grants to achieve this result.
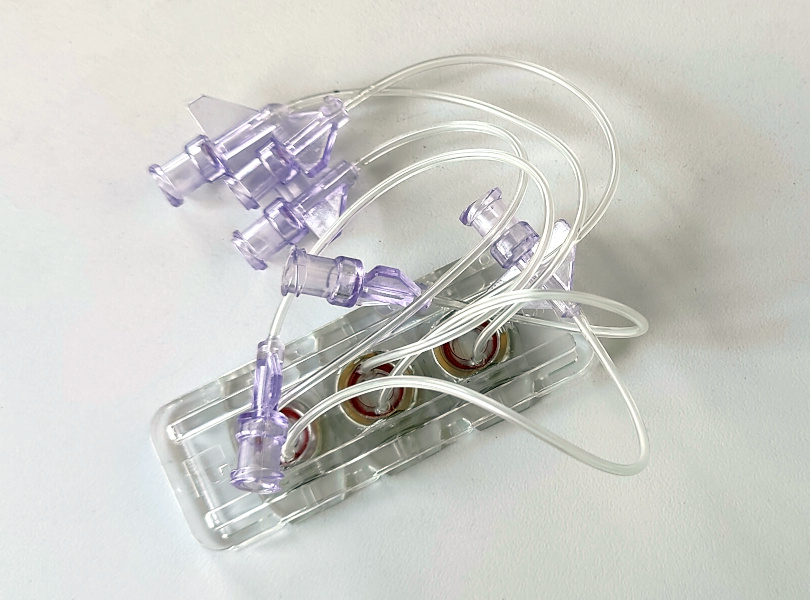
We’ve also worked with the European Space Agency (ESA) which is funding us to explore the use of the nichoid to do biology experiments in microgravity. We’ve developed, together with the Italian Institute of Technology, a version of the nichoid written with a laser on a polymer, instead of on glass. The experiments aboard the International Space Station are on a glioblastoma multiforme, a very aggressive form of brain cancer; we use tumour cells disseminated on the nichoid and placed in a small perfusion cartridge. The experiment set-up is small, weighs little, and can be used as two-dimensional for the operator even though it is three-dimensional for the cells.
Let’s talk a bit about your career path… How was this passion for research born?
I graduated in mechanical engineering, and I then chose to do a PhD in bioengineering, and I have been a bioengineer ever since. I started at the end of my PhD with cell cultures and threw myself almost completely into biology.
I interpret the bioengineer according the more international meaning of the term, compared to that used in Italy. Abroad, in fact, bioengineers don’t distinguish between their expertise in engineering and their expertise in biology. Bioengineers are scientists halfway between biologists and engineers. In Italy, there is still a strict disciplinary control to ensure those who do bioengineering remain an engineer, and those who do biology take up biology or medicine. This certainly doesn’t improve the competitiveness of Italian bioengineers in obtaining ERC funding.
How difficult is it to be awarded ERC funding?
They’re very competitive grants. The ERC funding I’ve received, by no mean feat, were the two fundamental research projects, that is the Consolidator Grant for NICHOID, and the latest Advanced Grant for BEACONSANDEGG The two ERC grants for technology transfer were smaller projects, easier to get.
In 2012 I submitted my first request for the NICHOID project, they didn’t fund me because, according to the assessors, I didn’t have sufficient scientific independence in the biological aspects of research.
I wanted this funding so much, I wanted to make myself independent and start my own mechanobiology laboratory. And so I enrolled in medicine. At the time I was 44. I did the admissions test with 18 year olds, I passed, I took all of the courses and took the various first year medicine exams here in Milan.
After that, I couldn’t continue because medicine requires mandatory attendance, something which was incompatible with my academic commitments. That first year, however, served me well in filling in all the gaps I had.
I resubmitted the project and finally they funded me for it.
Do you feel more like a biologist or an engineer?
Based on my expertise, I truly feel halfway between the two. However, my mentality is a bit more that of an engineer. I want to design, technology really interests me.
However, I understand the biologist’s mentality, similar to that of a chemist, in which extreme complexity must be confronted, and there is a dependence on hundreds of parameters, in all phenomena. That’s difficult for an engineer to deal with. Usually we have a few, well-defined parameters to control.
In biology, however, the phenomena are very complex, therefore you have to be able to simplify, to reduce the number of parameters and to open small, simplified windows of observation in phenomena of overwhelming complexity. We are only now beginning to incorporate theoretical research models.

That’s kind of what you do with your project…
Exactly. We open a window on a very controlled object because we made it ourselves, we put in the cells that we want, with a specific genetic mutation that we decide.
There can be many different mutations which produce the same type of tumour: we can control the type of cells that we use, the type of tumour that we implant in the living organism, and we can, with a microscope and the beacons, follow the evolution and construct a theoretic model of the fibrosis phenomenon.
It’s a classic bioengineering project, that is, engineering of a structure, generated by the engineer, outside of the human body, which comprises of an artificial and a cellular part. In this case, we created a model which combines an artificial part and even an entire living organism.
We open a microscopic window on a world of overwhelming complexity, in which we are able to control a single aspect, which is that which we are interested in at the moment.
Then, about my mentality, I truly become a hybrid between an engineer, who controls a system that they have designed, and a biologist, who also takes into account the much more complex system that influences the controlled part.
What stage is biology in today, and how is the relationship with engineering changing?
Biology and medicine are two disciplines which are, in this moment in history, in a transition phase: between being completely phenomenological – that is, I do an experiment, I tell you what happened, I do another experiment and I tell you what happened in this other case – and a vision which, instead of being model-based, is more theoretical – that is, I make a model, I write an equation, then I use an experiment to check whether the model provides correct predictions.
It’s an important moment for bioengineers because they put their capacity to make models and to control them at the service of this transition through which the discipline is going.

Our magazine is about research, it’s not by chance it’s called ‘Frontiers’. You have also dubbed your project a frontier project. Could you please explain us why?
It is truly a frontier project in the field of cellular modelling research. It’s neither basic research nor applied research: we are paving the way for a new approach to biological research. And a new frontier.
Today, the distinction between basic research and applied research, however, is limiting, in my opinion. I believe that there only exists basic research and its applications. But we need the results of basic research for ‘something’ to be applied.
With this project, we are mainly focusing on basic research. No one, as of yet, has ever made a fibrosis model, because it cannot be done in vivo nor in a laboratory. We had the idea to try a third way, a controllable hybrid as a laboratory model, but realistic like an animal model.
Something that doesn’t yet exist, but is now up to us to develop and authenticate.
What does the future hold for BEACONSANDEGG?
For the first five years we will be completely engrossed in this project. All of the lab researchers are enthusiastic, not least because their preliminary results, which they have achieved with great effort over these last years, were fundamental in receiving the funding.
We’re dealing, in fact, with a very risky project, but the fact that we have these preliminary results in hand reassures us. ERC projects are like this: ‘High risk, high gain’ The funding received is our fuel to fly high.
